- Magnetic Resonance Imaging Acquisition, Post-processing, Analysis, Computational methods
- Musculoskeletal PET-MRI (NaF) Imaging and Osteoarthritis research: 3.0T & 0.55T
- Disease Progression, Follow-up: T1p, T2, Bone, Cartilage, Muscle, Menisci health, compositional, metabolic and morphological Biomarkers in Osteoarthritis
- Machine Learning & Deep Learning: synthetic imaging, grading, image assessment, anomaly detection, denoising and segmentation
- Working and curating larger image databases, conducting and evaluating Health-Data specific AI challenges for Conferences (Miccai-2022)
- Experience working with Biomechanical data analysis (kinetics, kinematics, gait analysis)
- Exploring the technical feasibility of utilizing new-generation MRI scanners (0.55T) for Osteoarthritis evaluation
- Specialized in Neuro MRI Imaging Evaluation: Glioma & Acute Ischemic Stroke
- Susceptibility Weighted Imaging (SWI) and its utilization in novel scopes

Supporting science and clinical trials with advanced image analysis technology
Our Journey
learn more...
Our Expertise
learn more...
Our Motivation
learn more...
Our Vision
learn more...
Our Journey
Chondrometrics was founded in Munich in 2003 by Prof. Felix Eckstein and Prof. Reinhard Putz. Today, the company is headquartered in Freilassing, Germany – on the German–Austrian border near Salzburg.
Chondrometrics is a leading provider of medical image analysis for academia and industry, supporting both scientific research and clinical trials with advanced imaging technologies.
With more than 20 years of experience, we rely on an expert, quality-controlled manual image analysis pipeline that has received regulatory approval (see Our Expertise ). This approach has recently been supplemented by a fully automated analysis method (see Our Vision).
We use state-of-the-art MRI techniques to quantify structural changes in articular tissues. Our methods are applied in academic research as well as in clinical trials of pharmacological, surgical, or lifestyle interventions.
The overarching goal of our work is to qualify imaging biomarkers as surrogate endpoints in clinical trials – ultimately accelerating the development and approval of disease-modifying osteoarthritis drugs (DMOADs) and other innovative therapies.
Our Expertise
Chondrometrics provides regulatory-compliant medical image analysis services to pharmaceutical and medical device companies as well as academic institutions.
Our proprietary software platform, Chondrometrics 3.0, is a Class IIa medical device, certified under the current EU Medical Device Regulation (MDR 2017/745, Annex IX) (see certificate). All image analyses are conducted under a GCP-compliant quality management (QM) system, which is certified according to EN ISO 13485:2021 standards (see ISO certificate).
Both our QM system and software platform are audited annually and certified by Medical Device Certification GmbH (MDC), a notified body based in Stuttgart, Germany. We are also regularly audited by our industry partners and contract research organizations (CROs).
Our team of highly trained image analysts has more than 20 years of experience in manual segmentation of articular and musculoskeletal tissues (see Team ).
We are experts in the quantitative analysis of:
- Cartilage morphology (e.g. thickness, volume, surface area)
- Cartilage composition (e.g. T2 relaxation time)
- Meniscus morphology and position
- Bone, muscle, and adipose tissue mass
- Infrapatellar fat pad (IPFP)
- (Effusion) synovitis
Thanks to our in-depth anatomical expertise, we also offer high-quality analysis of other musculoskeletal structures and organ systems beyond the joint.
Our Motivation
Osteoarthritis affects more than 500 million people worldwide, making it a leading cause of pain and disability. It is associated with progressive structural changes in articular tissues that result in severe joint symptoms.
While early diagnosis and clinical management are crucial to maintaining patients' quality of life, no joint-structure-modifying therapy has yet been approved by regulatory agencies. Current treatments focus on pain management, while the modification of structural disease progression remains an unmet medical need.
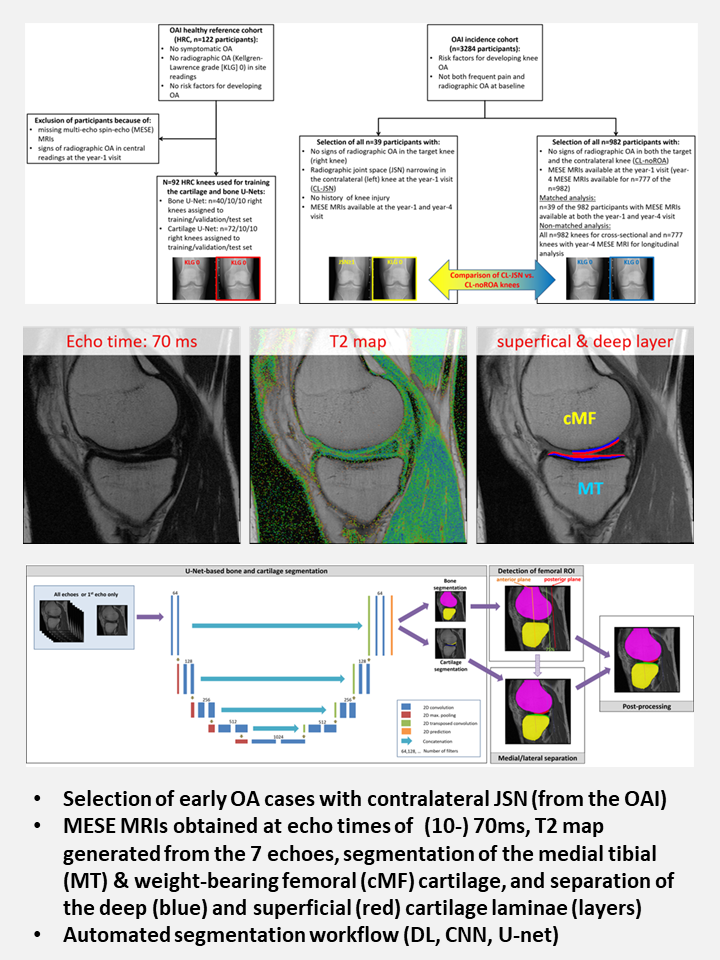
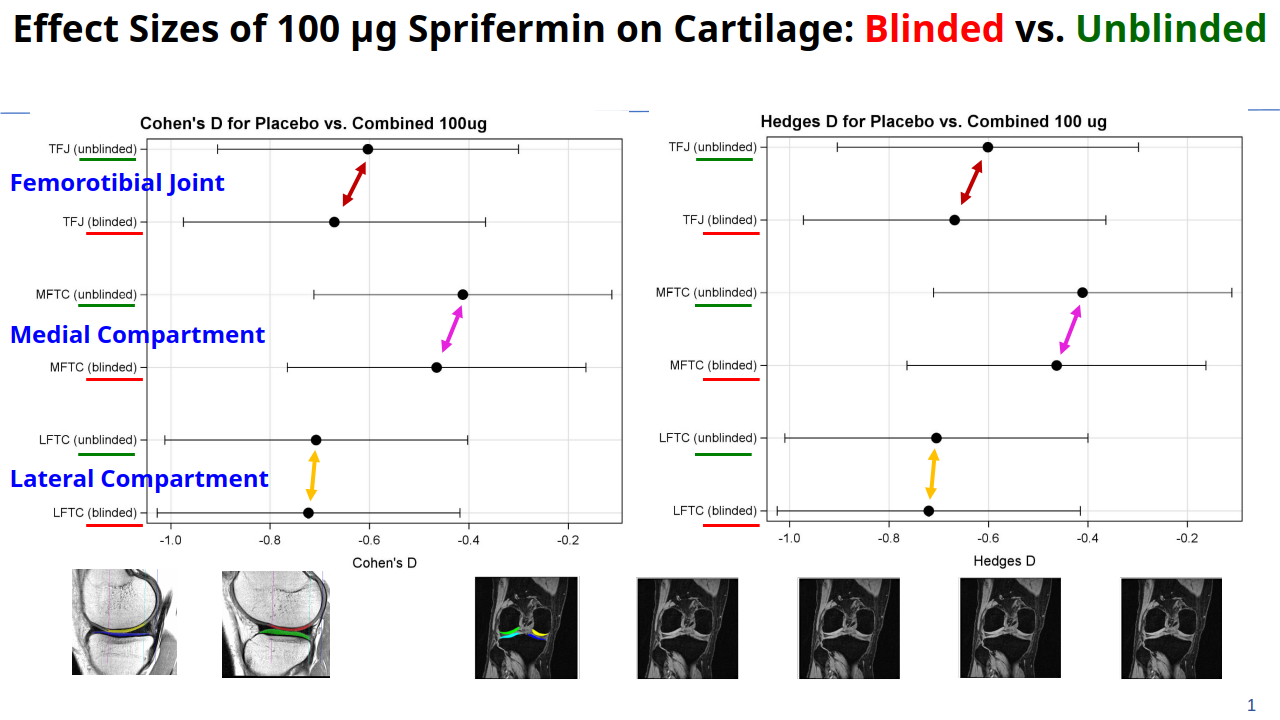
Structural pathology is best captured through imaging: small increments of tissue change can be precisely detected using quantitative techniques. Chondrometrics' validated methods can measure both disease progression and the effects of disease-modifying interventions with high accuracy and precision.
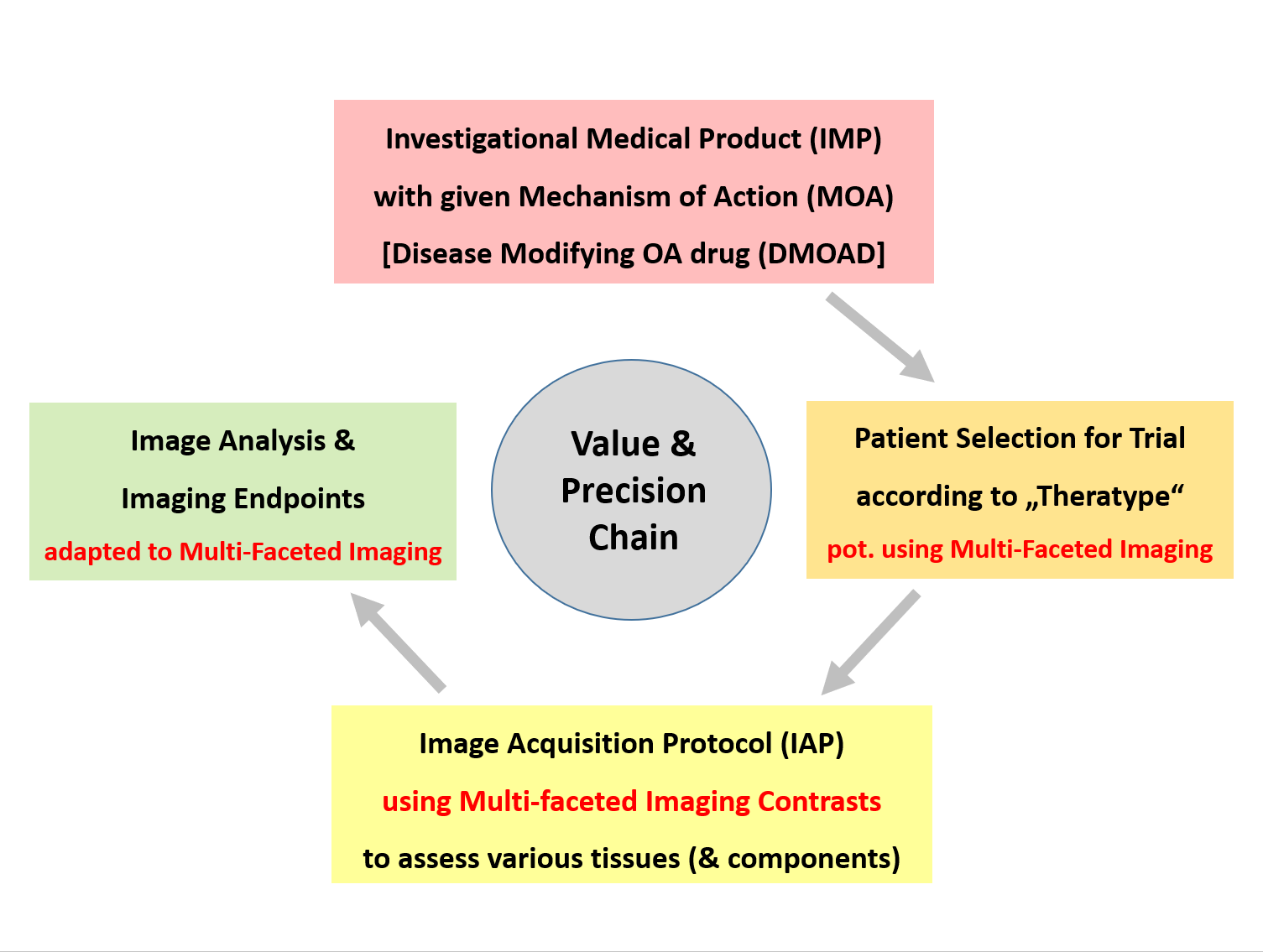
Our imaging-based approach also ensures that patient selection, imaging protocols, analytical methods, and endpoints are aligned with a drug's mechanism of action (MOA) – a critical requirement for the success of clinical trials.
From Phase I to Phase IV randomized controlled trials (RCTs), our methodology supports the pharmaceutical and medical device industry in testing the safety and efficacy of novel compounds aimed at modifying disease progression.
Specifically, we offer consultation on the optimal design of imaging acquisition protocols — ensuring that biomarkers, patient selection, and endpoints are fully aligned with the mechanism of action (MOA) of the investigational product. This alignment is crucial to generating conclusive and regulatory-relevant trial outcomes.
Our Vision
Chondrometrics is committed to developing precise, sensitive imaging technologies that make clinical trials more efficient, cost-effective, and conclusive.
Our vision is to establish quantitative imaging biomarkers not only as secondary endpoints but as regulatory-accepted surrogate markers that can replace clinical outcomes – directly supporting regulatory approval of medicinal investigational products (MIPs) as drugs.
To this end, we now offer a fully automated image analysis service based on a validated, efficient combination of deep learning (AI) and post-processing techniques.
These advances are grounded in more than 30 years of academic expertise in musculoskeletal imaging and clinical research.
Our automated technologies are trained and validated using a large resource of manually segmented datasets, ensuring both technical reliability and clinical relevance. This stringent alignment enables trials to produce clear, unambiguous answers about a compound's structural effects on osteoarthritis.
Interested to learn more and hear about the research done by company representatives.
Please visit the RESEARCH Section on this page. Contact: eckstein@chondrometrics.de















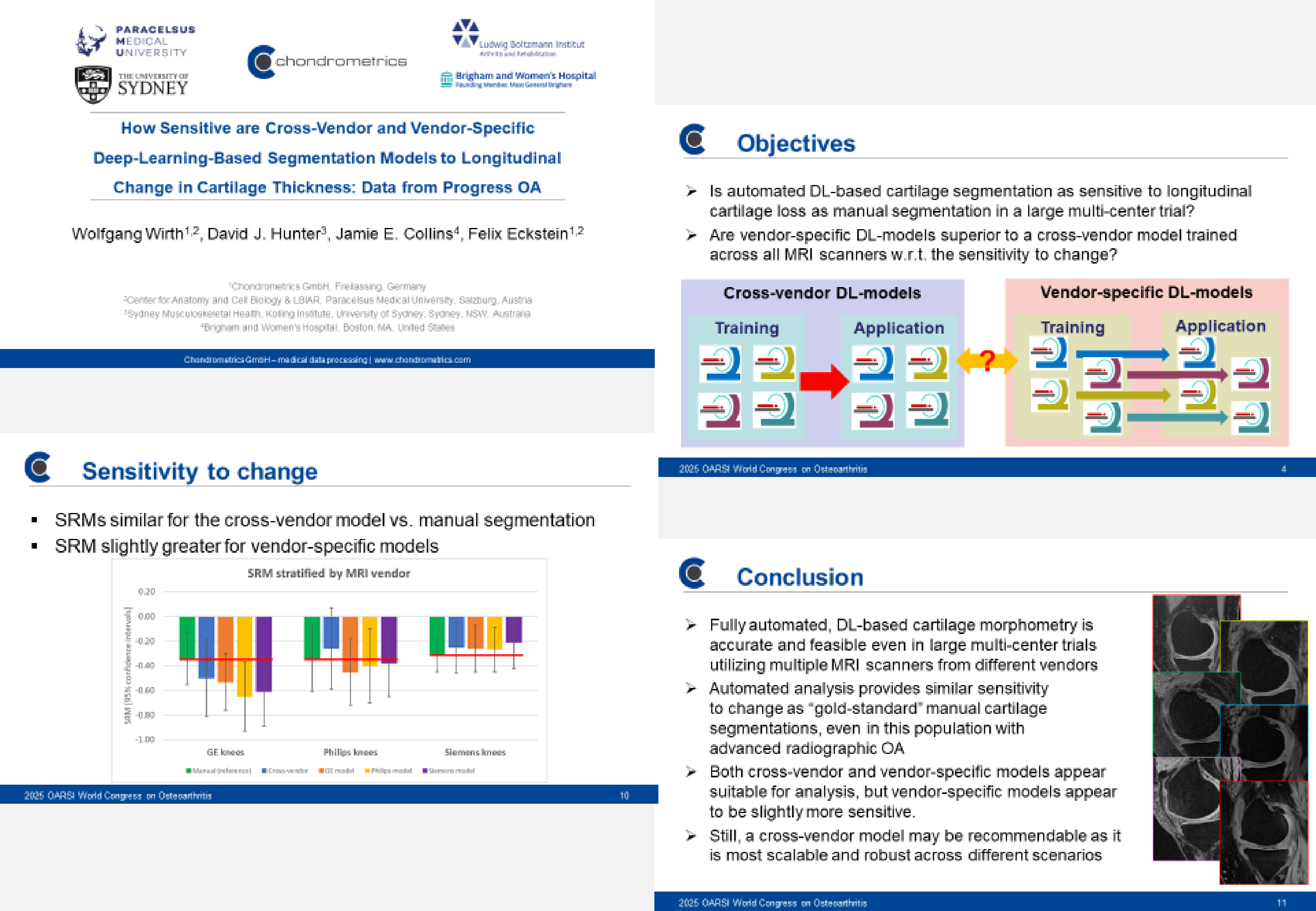


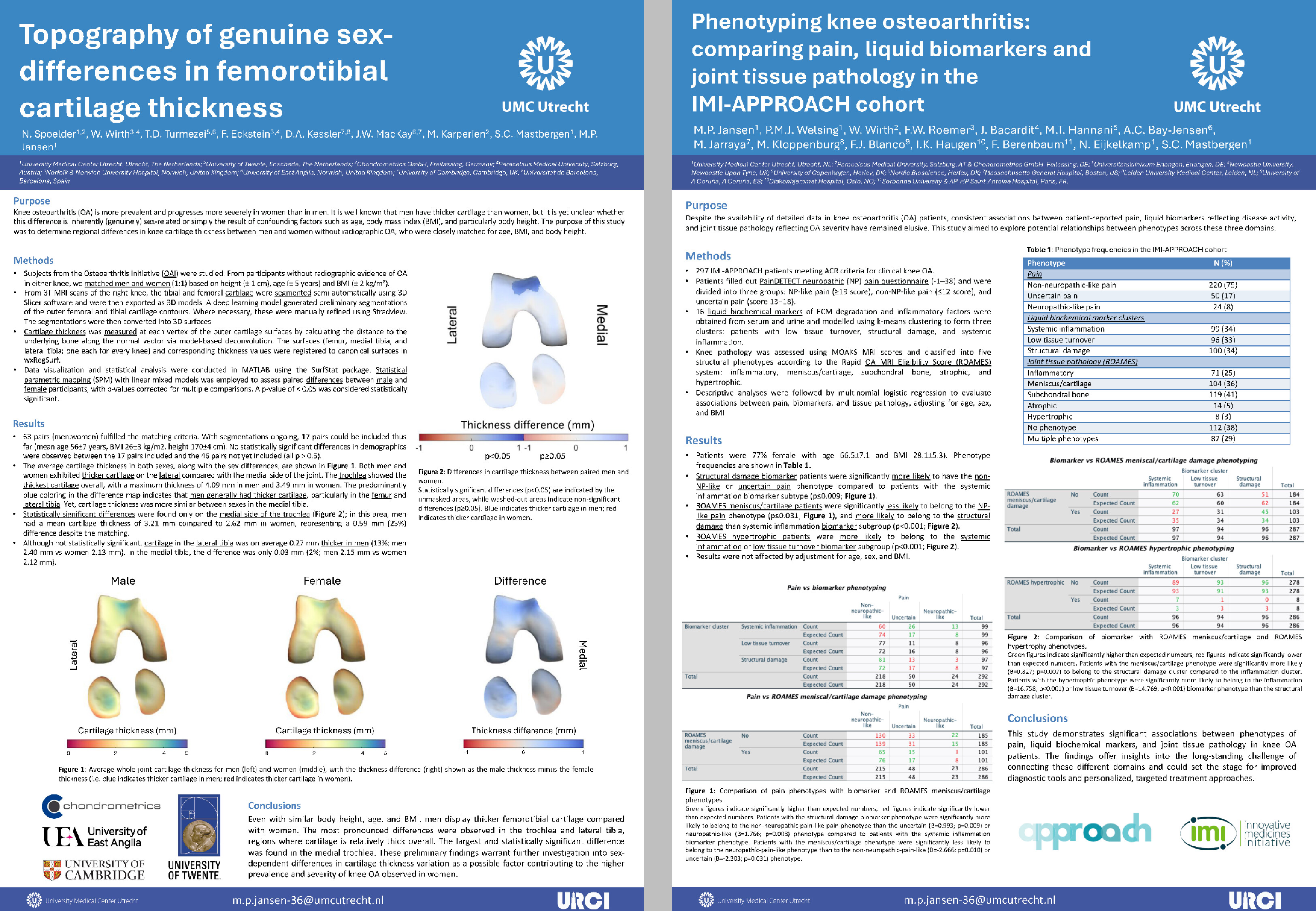



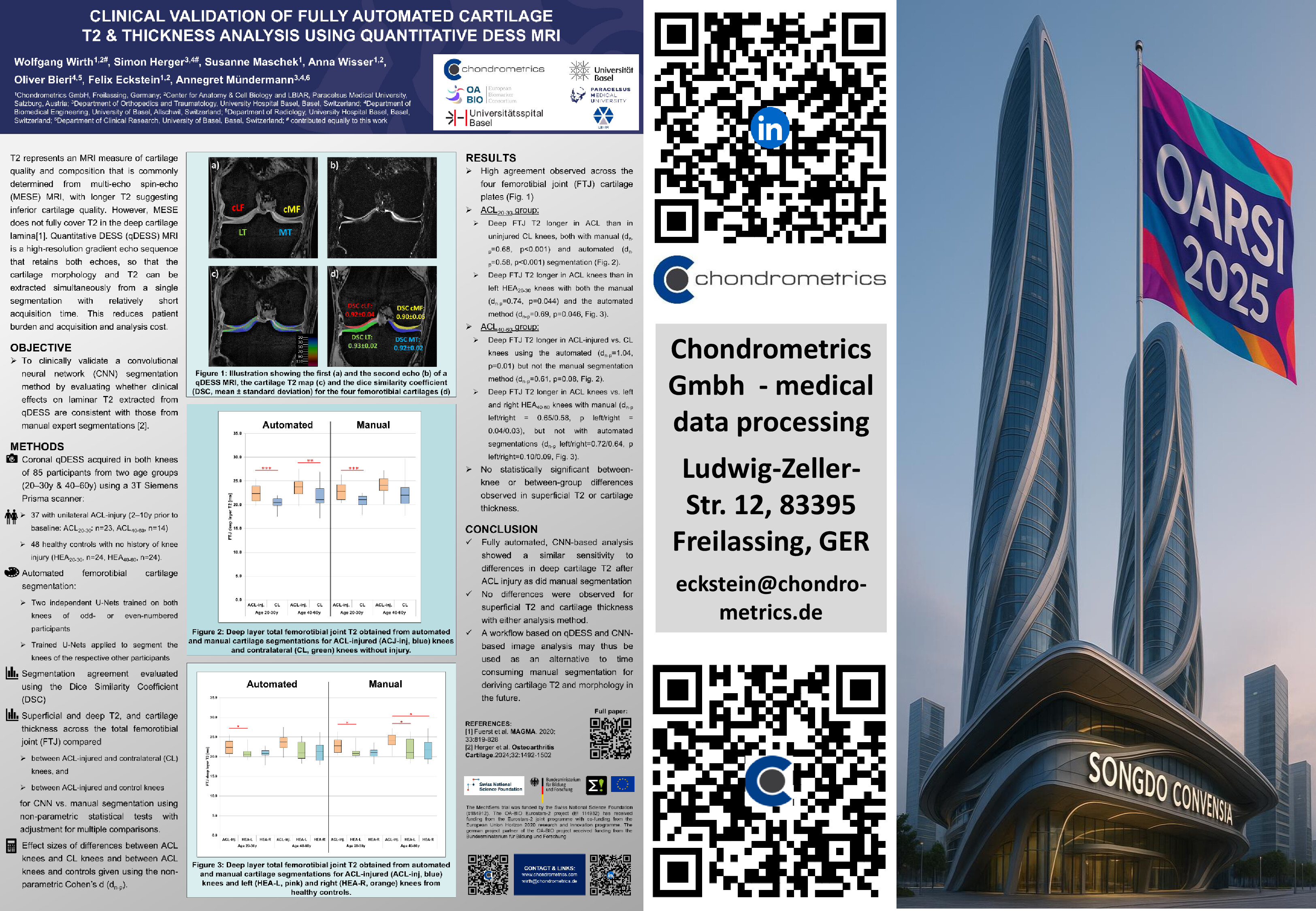
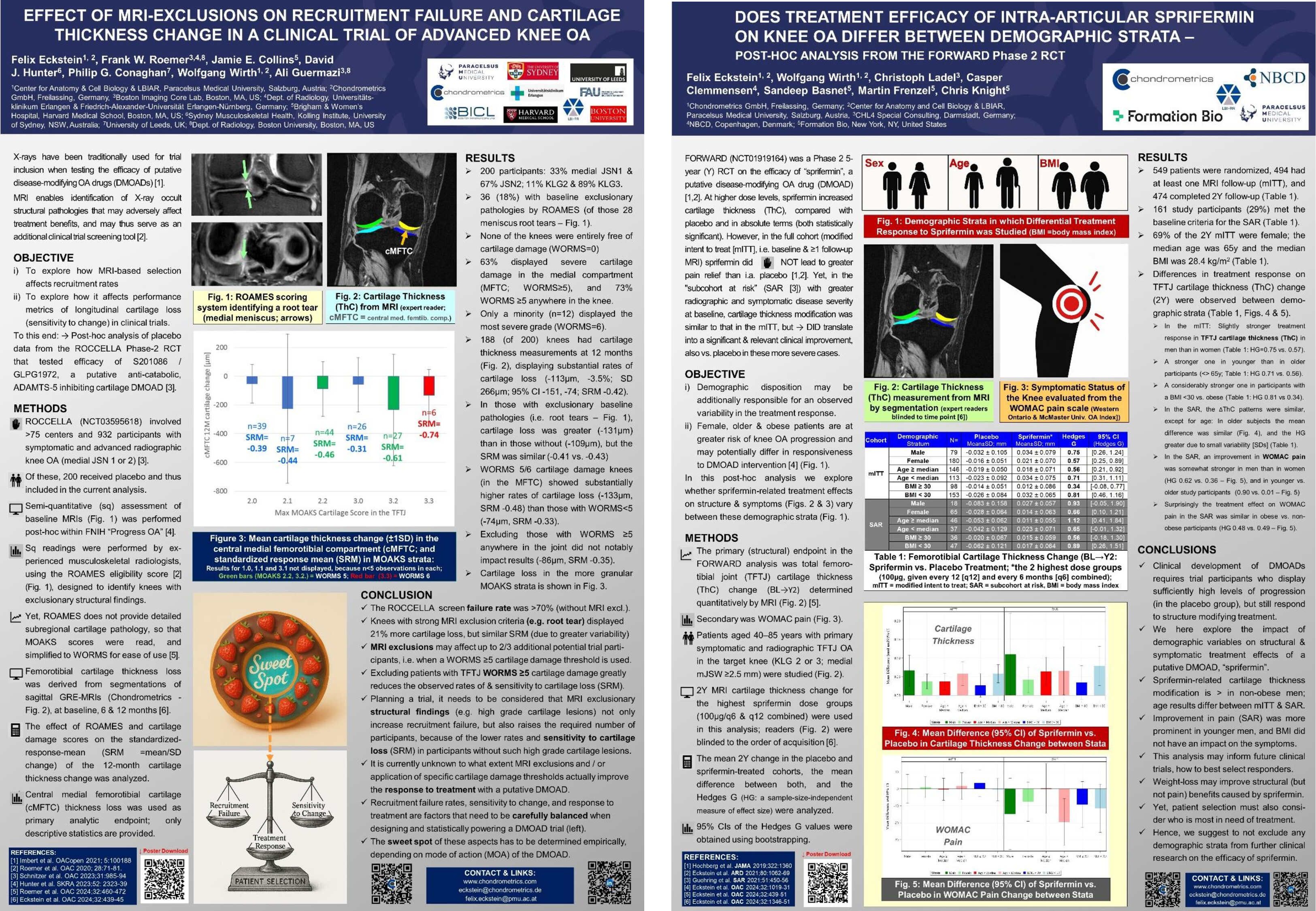










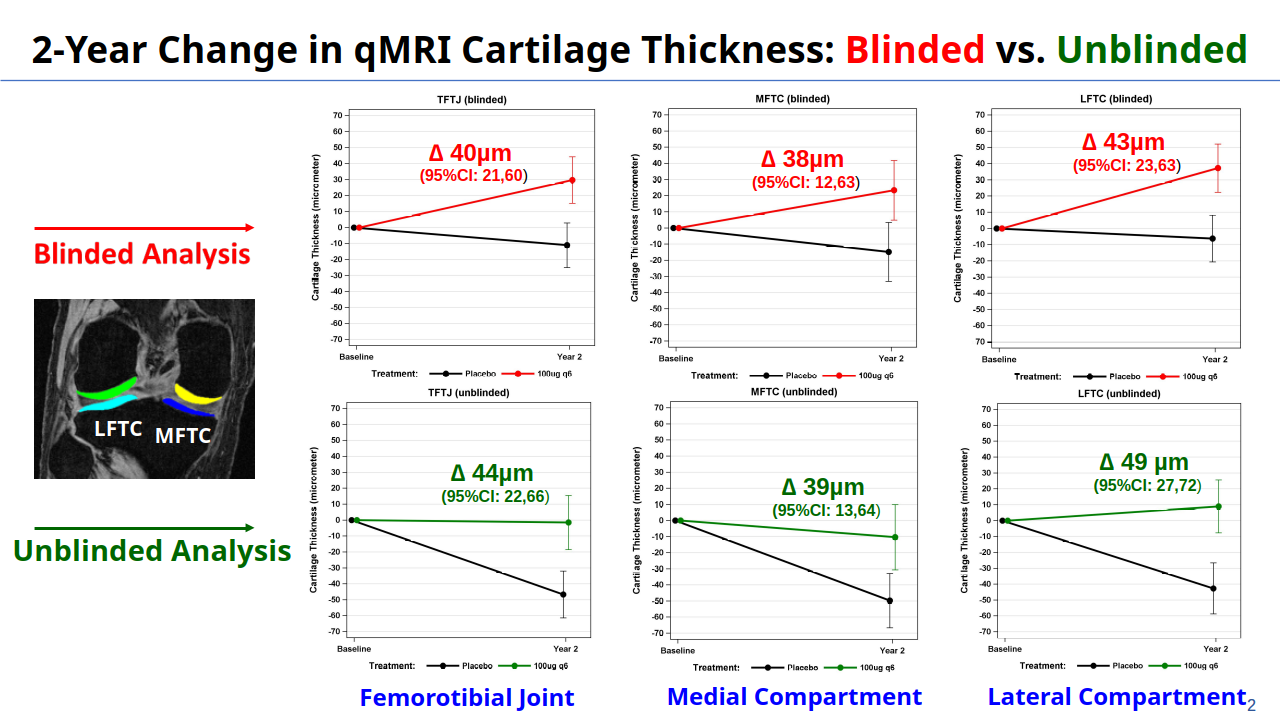
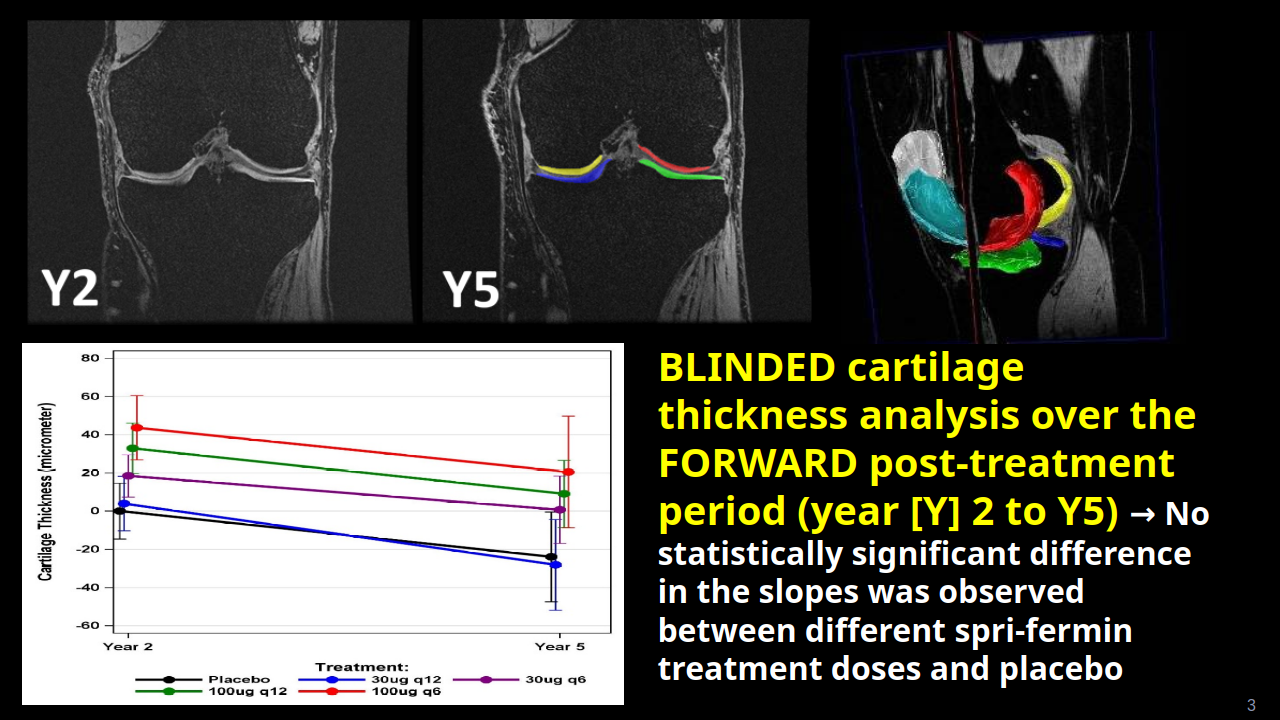
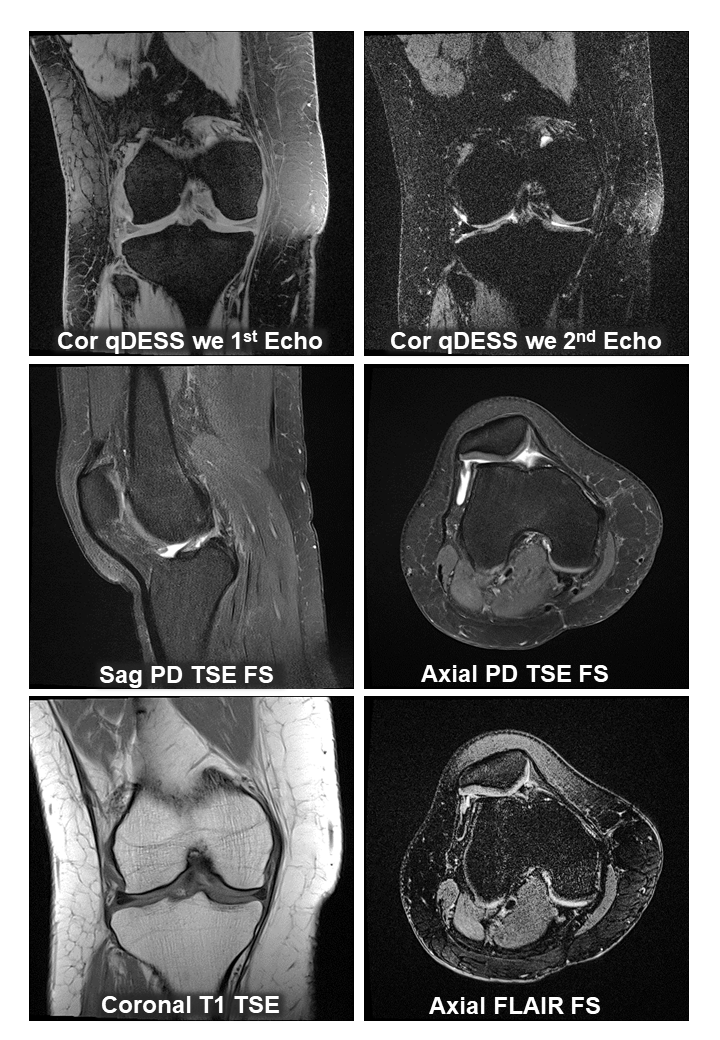



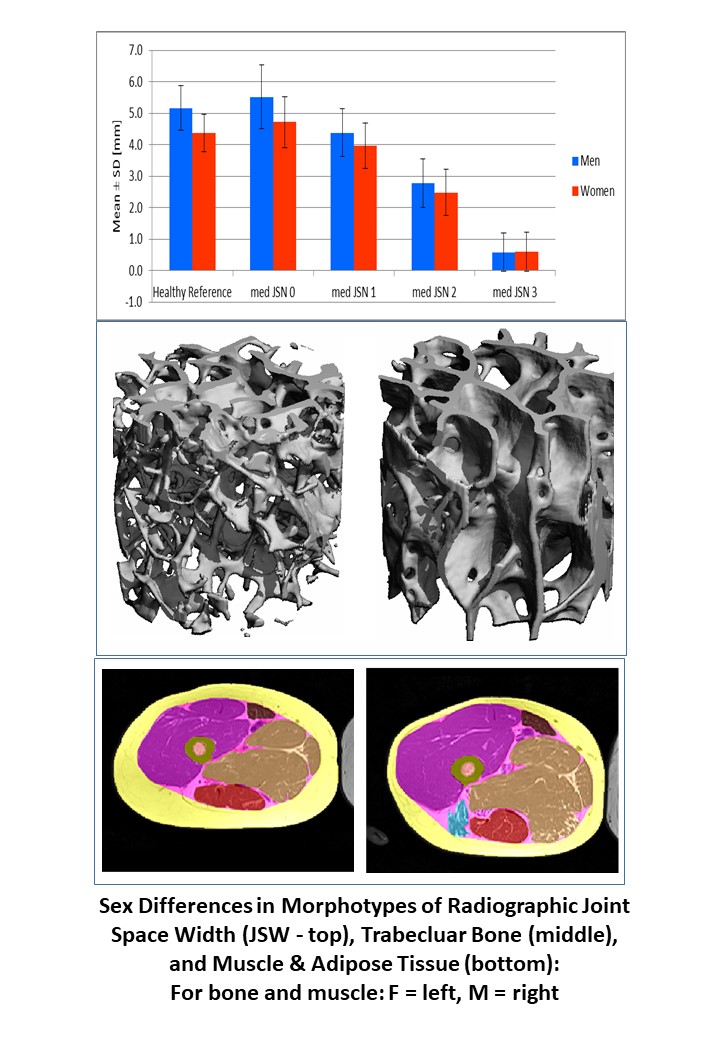


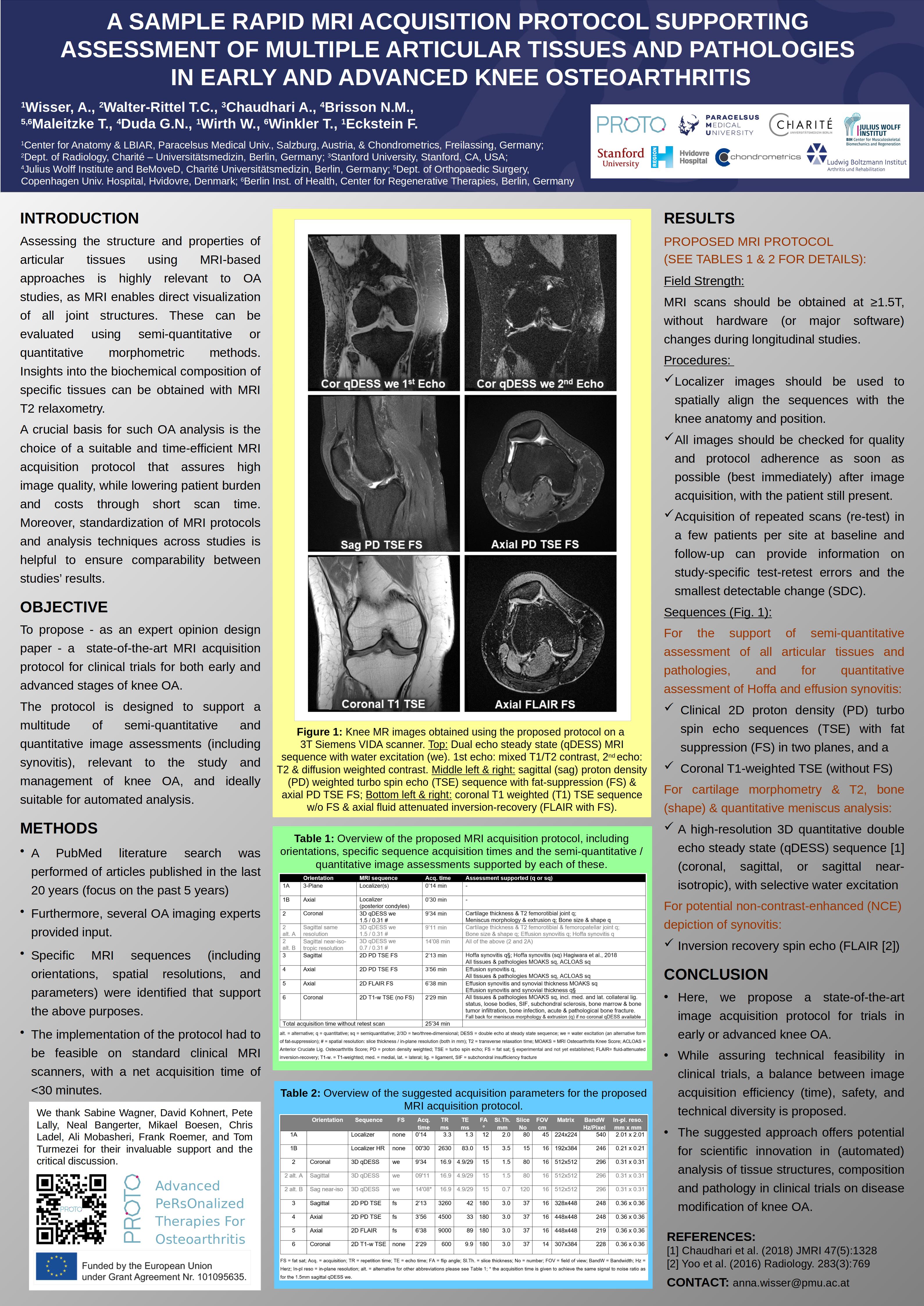



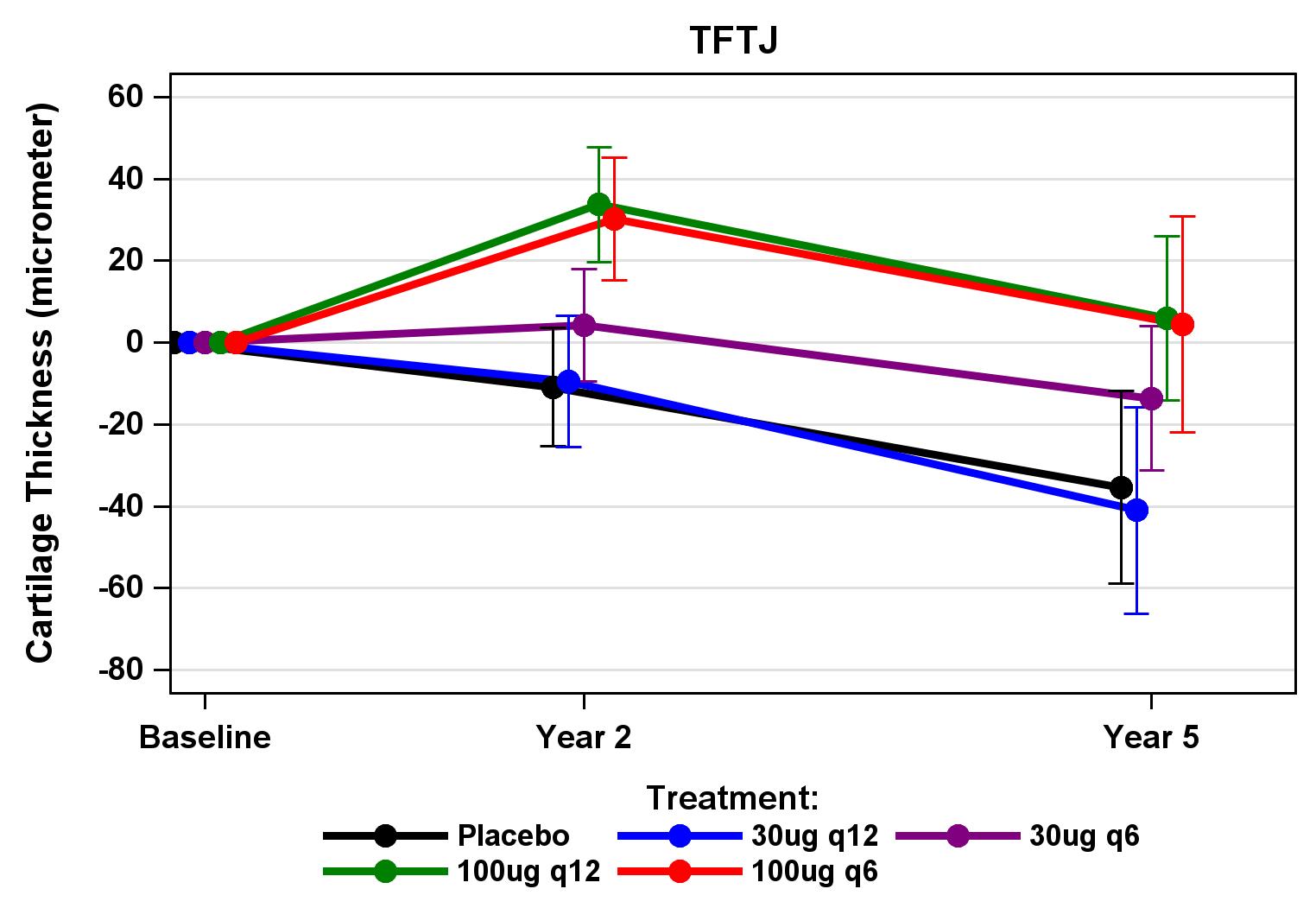

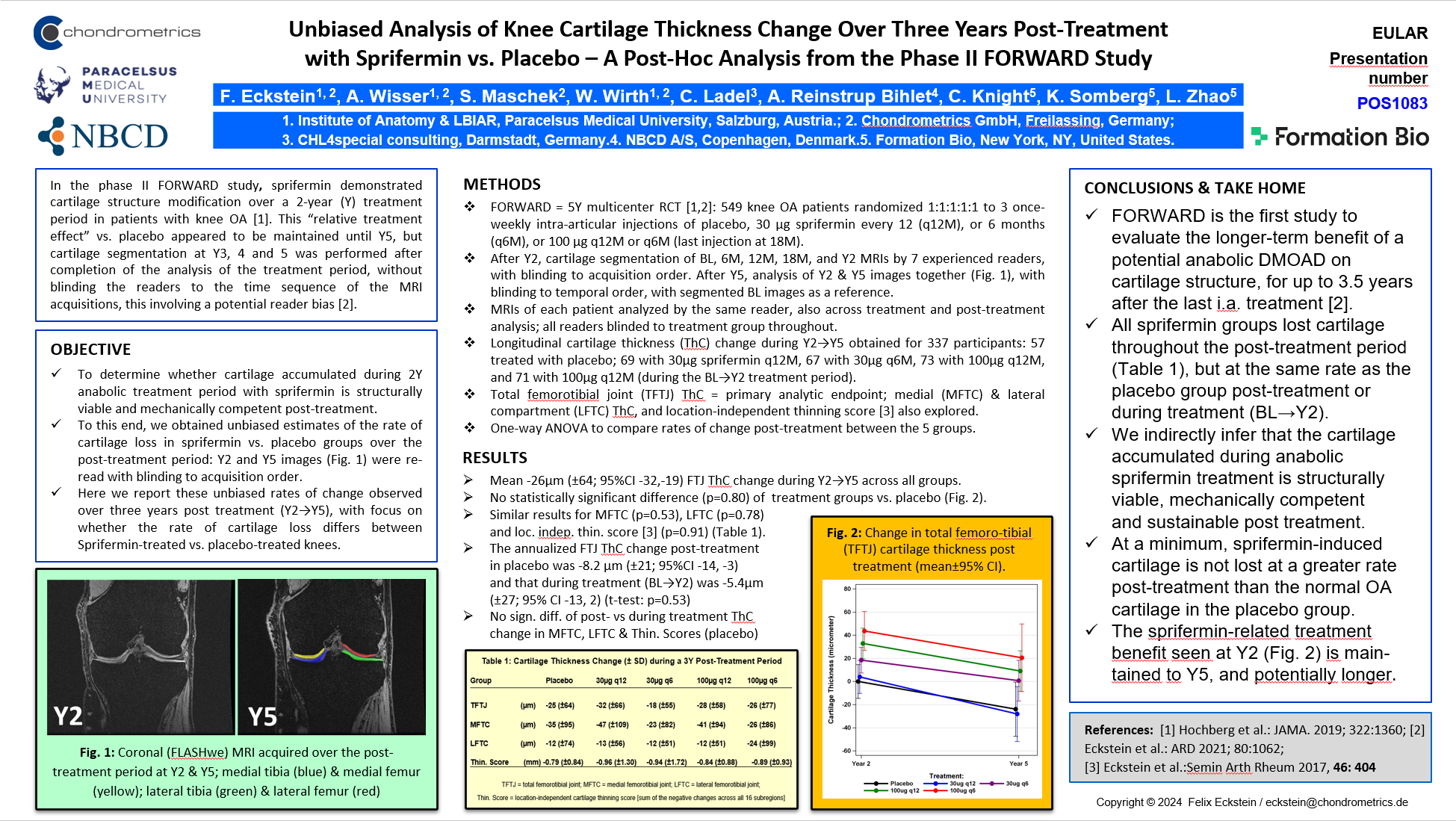







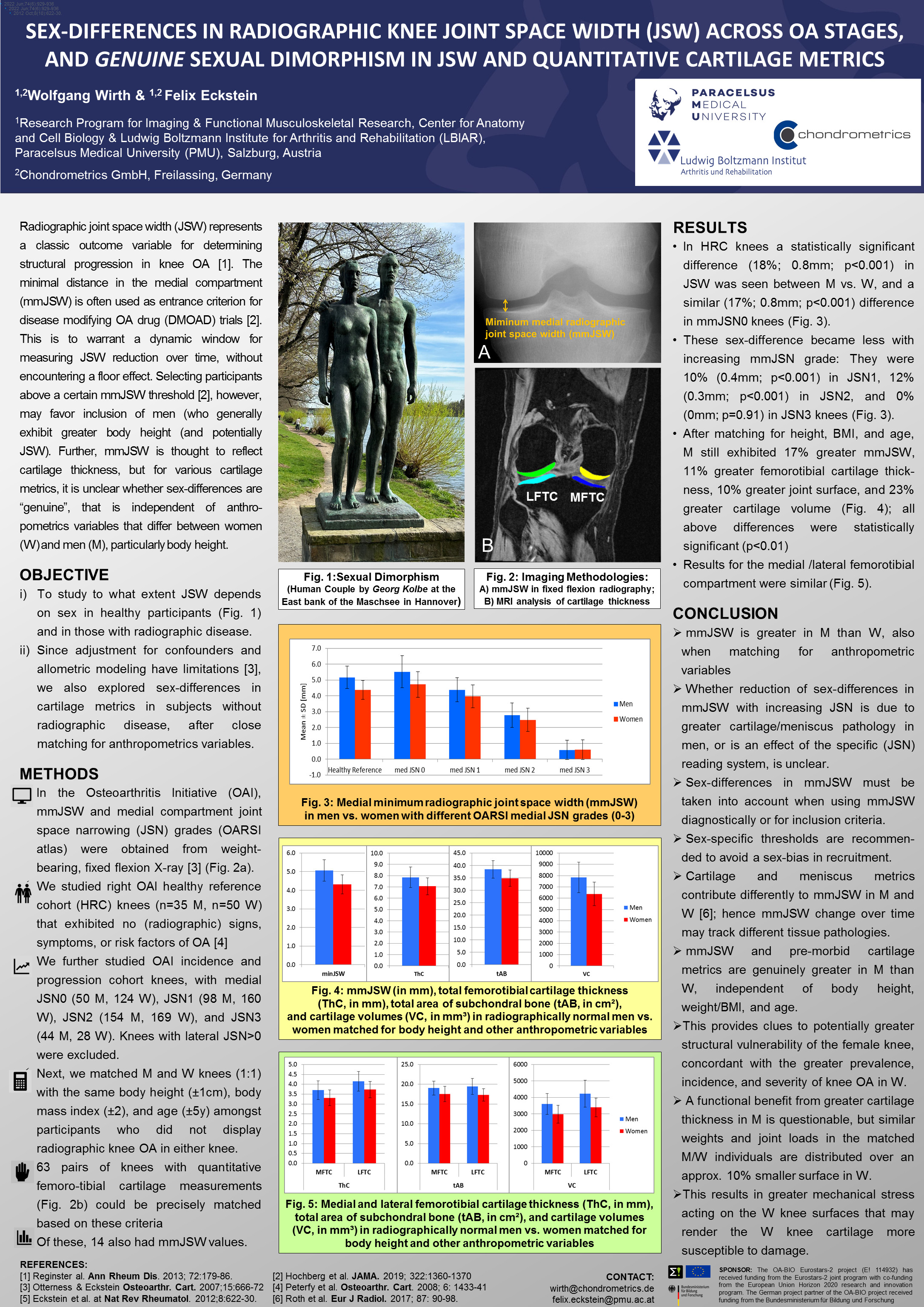




















 Chondrometrics GmbH provides expert consultation and image analysis service to academic researchers and the pharmaceutical industry. The strength of Chondrometrics, we believe, lies in the dedication and experience of its team of specialized researchers and readers, the profound integration with the scientific community, the rigorous scientific validation of its methodologies, and the transparency of its technology by publication in leading scientific journals. At Chondrometrics, we value our clients as collaborators, react immediately to their requests and specific needs, provide validated scientific information, communicate promptly, and act with anticipation.
Chondrometrics GmbH provides expert consultation and image analysis service to academic researchers and the pharmaceutical industry. The strength of Chondrometrics, we believe, lies in the dedication and experience of its team of specialized researchers and readers, the profound integration with the scientific community, the rigorous scientific validation of its methodologies, and the transparency of its technology by publication in leading scientific journals. At Chondrometrics, we value our clients as collaborators, react immediately to their requests and specific needs, provide validated scientific information, communicate promptly, and act with anticipation.